 1
1
(S)-3-Hydroxypyrrolidine, also known as (S)-3-吡咯烷醇, is a chiral pyrrolidine compound that is a light yellow transparent liquid at room temperature and pressure. It exhibits significant alkalinity and sensitivity to oxidants, with some solubility in water and common organic solvents. Primarily used as a chiral amino alcohol in organic synthesis and pharmaceutical intermediate, (S)-3-Hydroxypyrrolidine has been reported to be involved in the preparation of pyrrolidine platelet aggregation inhibitors through chemical transformations of its alcohol hydroxyl group.
The nitrogen atom on the pyrrole ring unit of (S)-3-Hydroxypyrrolidine possesses certain alkalinity and nucleophilicity, allowing it to undergo acid-base neutralization reactions with common acidic substances. Furthermore, (S)-3-Hydroxypyrrolidine can undergo condensation reactions with organic aldehydes to yield corresponding enamine derivatives, which are utilized in catalyzing various asymmetric condensation reactions.
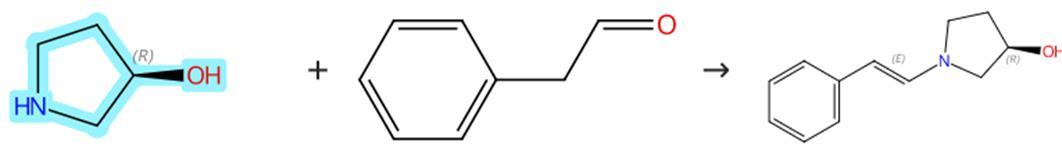
Figure 1: (S)-3-Hydroxypyrrolidine's condensation reaction
In a dry Schlenk tube, anhydrous sodium sulfate (4.26 g, 30.0 mmol, 15.0 equiv), anhydrous dichloromethane (5.0 mL), and freshly distilled 2-phenylacetaldehyde (240 mg, 2.0 mmol, 1.0 equiv) are combined. Freshly distilled (S)-3-Hydroxypyrrolidine (174 mg, 2.0 mmol, 1.0 equiv) is then slowly added dropwise under a nitrogen atmosphere. The resulting reaction mixture is stirred at room temperature for about an hour, and the reaction solution is transferred to a dry NMR tube through a drying syringe to determine the yield of the reaction system by NMR. After the reaction, the reaction mixture is concentrated under vacuum to remove the organic solvent, and the residue can be used directly for the next chemical reaction without further purification.
As a chiral amino alcohol, (S)-3-Hydroxypyrrolidine finds extensive applications in organic synthesis, serving as a starting material for the synthesis of various drug molecules. By undergoing condensation reactions with organic aldehydes to form enamine derivatives, (S)-3-Hydroxypyrrolidine is commonly employed in catalyzing asymmetric condensation reactions due to its chiral nature, resulting in products with excellent stereo selectivity that are crucial for the synthesis of pharmaceuticals and fine chemicals.
[1] Mohiti, Maziar; et al Chemistry-A European Journal 2024,30,e202303078.